Update from author (October 2017): New hydrogen spectroscopy results, with terrific precision, were published at long last on October 6. Before these data, all measurements made with hydrogen agreed with each other, but strongly disagreed with muonic hydrogen spectroscopy. Now, the tables have turned. The new spectroscopy is consistent with what looked to be recalcitrant muonic hydrogen results, but now in disagreement with all the previous hydrogen results. Attention will now be turned to all the other hydrogen measurements. Were there errors in the analysis? To say the least, those of us pulling for exotic physics to resolve the puzzle have suffered a strong blow.
Size matters. The proton, for instance, has a radius a tad less than one femtometer—one billionth of one millionth of a meter. Figuring out exactly how much less than one femtometer the proton’s radius is has stirred up one of the greatest controversies in all of modern physics, one we deem the “proton radius puzzle”. While the puzzle might be resolved by something mundane, it may also be our strongest clue toward understanding dark matter or marrying gravity and quantum mechanics.
At the heart of the puzzle sits a group of vexingly discordant measurements. With their ultra-fine precision, it has become clear that these measurements tell very different stories about the size of the proton. The situation is so dramatic that CODATA, the international authority on values of fundamental constants, refused to update the accepted value in their most recent round of recommendations, citing the “severe disagreement” among the measurements. The clashing measurements come from three separate methods: tossing electrons at protons and watching how they scatter outward, using hydrogen spectroscopy to investigate the quantum mechanical orbits of an electron as it flies around a proton, and, similarly, using muonic hydrogen spectroscopy.
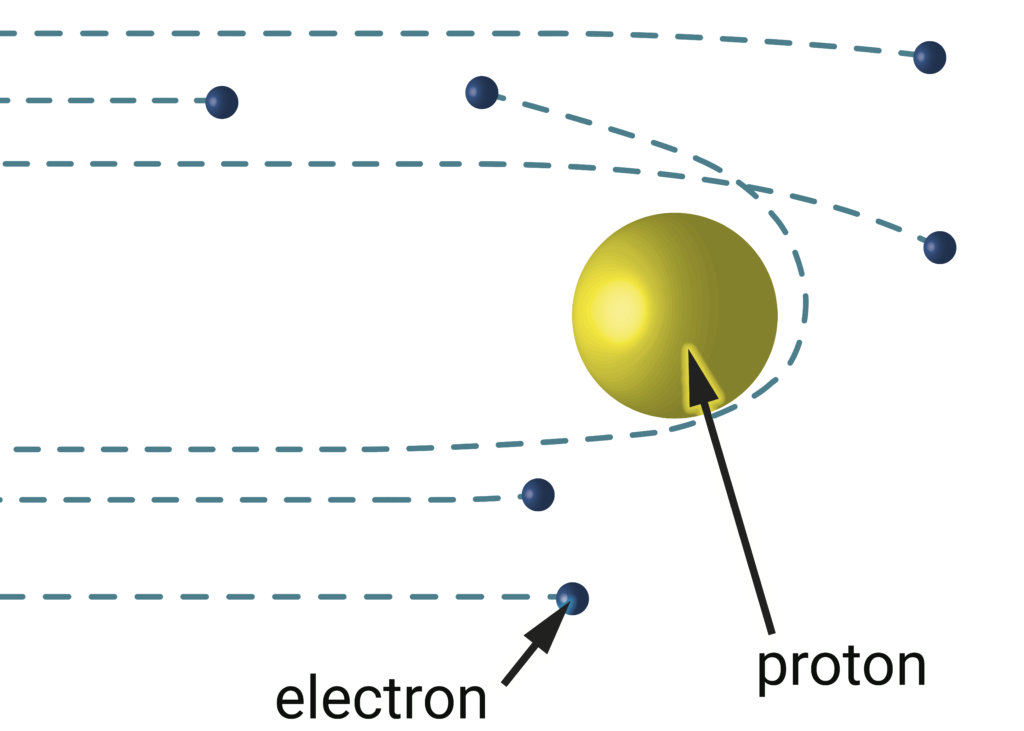 In electron-proton scattering experiments, electrons (blue) bombard the proton (yellow). Since the particles are oppositely charged, the electrons are attracted to the proton and bend toward it, often leaving at glancing angles. Rarely, when the electrons approach closely enough, it will look like they bounced out at backward angles. The proton’s size can be inferred by mapping the angles into which electrons scatter. Image credit: Lauren Borja, BSR design team.
In electron-proton scattering experiments, electrons (blue) bombard the proton (yellow). Since the particles are oppositely charged, the electrons are attracted to the proton and bend toward it, often leaving at glancing angles. Rarely, when the electrons approach closely enough, it will look like they bounced out at backward angles. The proton’s size can be inferred by mapping the angles into which electrons scatter. Image credit: Lauren Borja, BSR design team.
In the scattering experiments, one simply fires a slew of electrons at the proton and records how they bounce off the proton. Imagine firing pellets at a tank. As the pellets strike different faces of the tank, they ricochet off at different angles. By measuring the angles at which those pellets get deflected by the tank, you can deduce the shape and size of the tank. These scattering experiments thereby provide a measure of the proton’s radius: about 0.88 femtometer. Scattering experiments provide more information the further they can zoom into the tiniest angles of deflection. That resolution can be difficult to achieve, but spectroscopy provides a route to fantastic precision.
The spectroscopy experiments are a little more subtle. Let’s start with hydrogen. A hydrogen atom is just a proton with a tightly associated electron. Being a quantum particle, the electron is more like a wave whose position you can’t exactly pinpoint. Imagine you and I were enjoying a concert (anything from punk to funk) and I asked you, “Where is the music? Where is the sound wave?”—you’d be rightfully confused. The wave is at many spots in the room all at once. Just like a wave, the electron is delocalized—it occupies a whole region of space at once. The electron’s quantum mechanical nature restricts it to “orbiting” the proton only in very specific shapes. We call those orbital shapes “states”. A unique family of states called s states actually allow the electron to penetrate into the space occupied by the proton. Other families of states, for example, p states, exclude the electron from snuggling up with the proton.
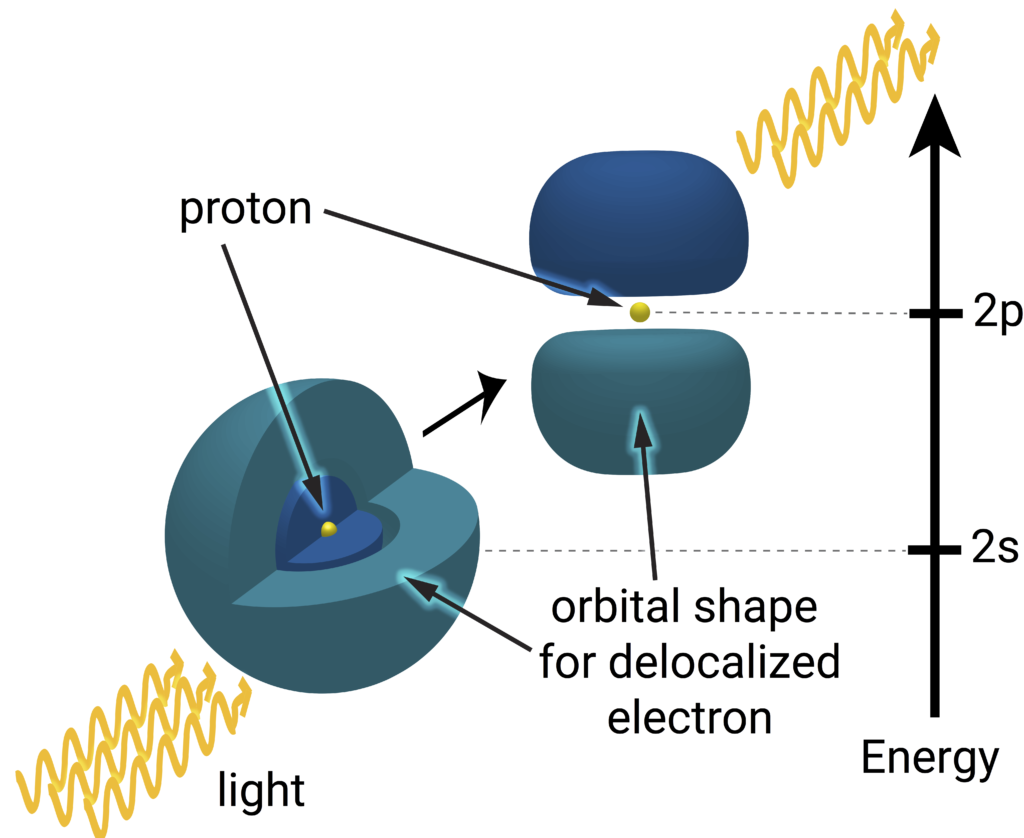 A hydrogen atom is nothing more than an electron (blues) bound to orbit a proton (yellow). Only light of a very specific energy can be absorbed by the hydrogen atom and inspire an electron to change its orbit. An atom in the s state absorbs one of the photons in order to be excited to the p state. An electron orbiting a proton can’t be localized to a point, in principle, so they are drawn as delocalized clouds. The dark and light blues representing the electron cloud signify opposite quantum phases, an important consideration for chemical properties, but have no consequence in the spectroscopy experiments. You might notice a curious void between the dark and light blues in the 2s state, which represents a “node”, a region where the electron will never venture. Since the s state overlaps with the nucleus (where the proton resides) but the p state does not, the energy difference between the states is in part determined by the size of the proton. Image credit: Lauren Borja, BSR design team.
A hydrogen atom is nothing more than an electron (blues) bound to orbit a proton (yellow). Only light of a very specific energy can be absorbed by the hydrogen atom and inspire an electron to change its orbit. An atom in the s state absorbs one of the photons in order to be excited to the p state. An electron orbiting a proton can’t be localized to a point, in principle, so they are drawn as delocalized clouds. The dark and light blues representing the electron cloud signify opposite quantum phases, an important consideration for chemical properties, but have no consequence in the spectroscopy experiments. You might notice a curious void between the dark and light blues in the 2s state, which represents a “node”, a region where the electron will never venture. Since the s state overlaps with the nucleus (where the proton resides) but the p state does not, the energy difference between the states is in part determined by the size of the proton. Image credit: Lauren Borja, BSR design team.
This difference in how close the electron in s and p states can get to the proton actually leads to a measurable difference in their energies. The effect is similar to a gravitational effect; if you were to bore towards the center of Earth, the gravitational force pulling you inward decreases the closer you get to the core. The more of Earth that’s behind you, the more that mass gravitationally tugs you in the other direction. In other words, the Earth holds onto you less strongly, or with a little less energy, at its core. In the same way, an electron in an s orbit penetrates into the core of the proton are held onto a little less strongly, or with a little less energy, than a p electron that does not penetrate. Precisely measuring the difference in energy between those s and p states via laser spectroscopy thereby gives a measurement of the proton’s radius. By measuring the difference in how strongly the proton holds onto the electron (the electron’s energy) between these states using laser spectroscopy, we can get a measurement of how big the proton is, so long as all other effects are properly taken into account. Hydrogen spectroscopy yields results consistent with the electron-proton scattering experiments, also suggesting that the proton has a radius of about 0.88 femtometer.
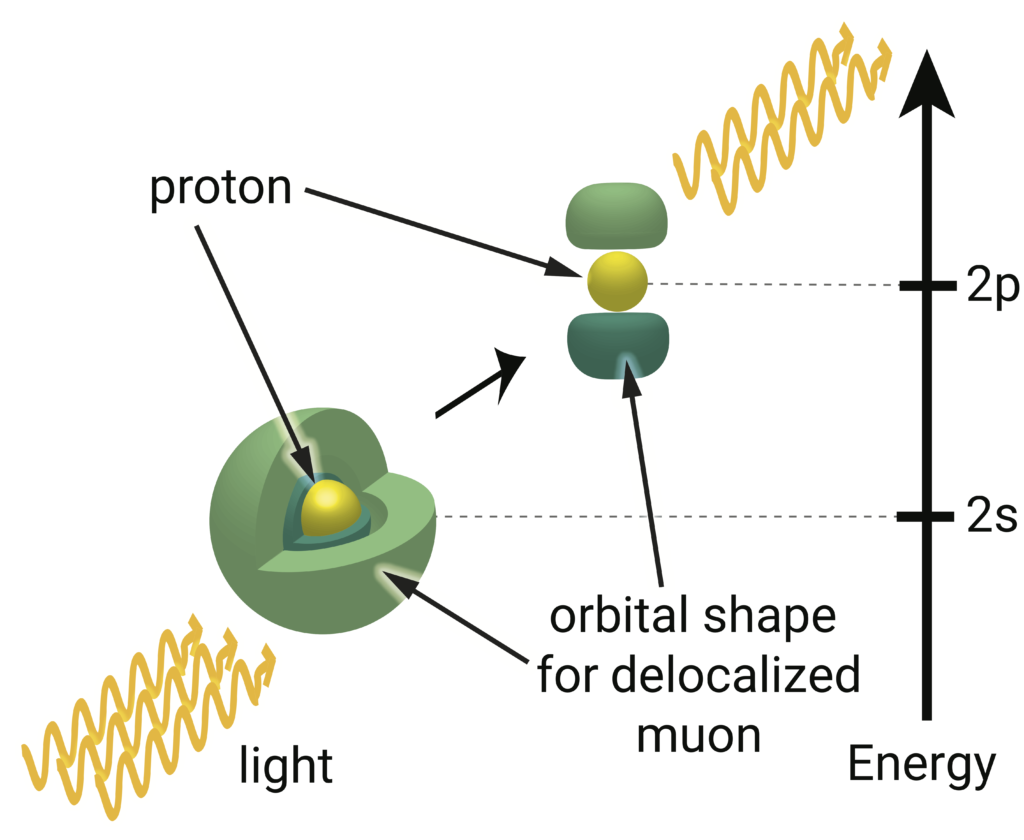 Muonic hydrogen looks just like hydrogen, but with a muon (greens) playing the role of hydrogen’s electron. Again, the s state overlaps with the proton (yellow), but the p state does not. Because it is heavier than an electron, the muon orbits much more tightly around the proton and overlaps more dramatically with the proton in its s state, permitting a more precise probe of the proton’s size. The proton is not larger in muonic hydrogen than hydrogen, but we have zoomed in on the proton in this figure compared to the hydrogen spectroscopy figure to emphasize how much more overlap there is between the muon and proton. Image credit: Lauren Borja, BSR design team.
Muonic hydrogen looks just like hydrogen, but with a muon (greens) playing the role of hydrogen’s electron. Again, the s state overlaps with the proton (yellow), but the p state does not. Because it is heavier than an electron, the muon orbits much more tightly around the proton and overlaps more dramatically with the proton in its s state, permitting a more precise probe of the proton’s size. The proton is not larger in muonic hydrogen than hydrogen, but we have zoomed in on the proton in this figure compared to the hydrogen spectroscopy figure to emphasize how much more overlap there is between the muon and proton. Image credit: Lauren Borja, BSR design team.
We can get a more precise spectroscopic measurement if we do the same experiment with one tweak. Instead of letting an electron orbit the proton as in hydrogen, we supplant the electron with a different particle called a muon, constructing what’s called muonic hydrogen. For all intents and purposes (as far as we know), the muon is identical to the electron except that it’s 200 times more massive. The bloated muon is more sluggish, so it falls into tighter orbits around the proton. That means the muon’s s states overlap even more with the proton than the electron’s s states, so the effect of sitting right on top of the proton is exaggerated for the muon. The muonic hydrogen results have delivered a measurement of the proton size with 10 times finer precision, but at a totally incompatible radius of closer to 0.84 femtometer, 4 percent smaller than what the hydrogen results yield.
The startling discrepancy prompted another set of spectroscopy experiments with an extra neutron—a charge-free particle that cohabitates with protons in the nuclei of all atoms except hydrogen—butted up against the proton. The proton and neutron together comprise a composite particle we call a deuteron. Stick an electron in orbit and call it deuterium instead of hydrogen, or stick a muon in orbit and call it muonic deuterium. Spectroscopic measurements with muonic deuterium were finally completed in August of 2016 and, here again, the results with a muon point to a significantly smaller deuteron radius than the results with an electron.
These results don’t just reaffirm the proton radius puzzle—they may even hint at surprising new physics. Despite doing essentially the same test with an electron and a muon, the proton radius looks distinctly smaller when you probe it with a muon. It could be the first sign of a new, hitherto undiscovered particle, one that affects muons differently than electrons. If such a new particle is to blame, it might also be culpable for the effects we attribute to dark matter and dark energy, two of the most prominent outstanding problems in physics. Some propose that the puzzle might be a hint of quantum gravity since the more massive muon would experience stronger gravitational interactions with the proton than would the puny electron. Alternatively, the puzzle might be resolved by something more mundane. For example, it could be that the spectroscopy data has been misinterpreted due to an error in the value of the Rydberg constant, which describes how strongly orbiting electrons and muons are bound to the proton. On the other hand, the Rydberg constant does happen to be exalted as our most precisely measured fundamental constant.
With such high stakes as to have seen the first direct hint of a dark matter particle, no effect that could possibly sully these ultra precise measurements can be left unexplored. Every stray electric field from a screw, every minuscule bug in the analysis code from a grad student long gone, every nefarious effect from special and general relativity, every parcel of heat emanating from a wall, must be identified, characterized, wrangled, and tamed. These systematic effects make precise measurements like these painstaking, but the potential payoff of revealing new physics is worth it. That's why a group at the Max Planck Institute of Quantum Optics in Germany is performing even more hydrogen spectroscopy measurements. In fact, at a Precision Physics conference in May 2017, they presented preliminary results from a new experiment very similar to the 2s-2p spectroscopy described above. While the hunt for systematics will continue until publication, the forthcoming results and their implications for the proton radius will be very interesting, to say the least (stay tuned for updates on when their paper is officially published).
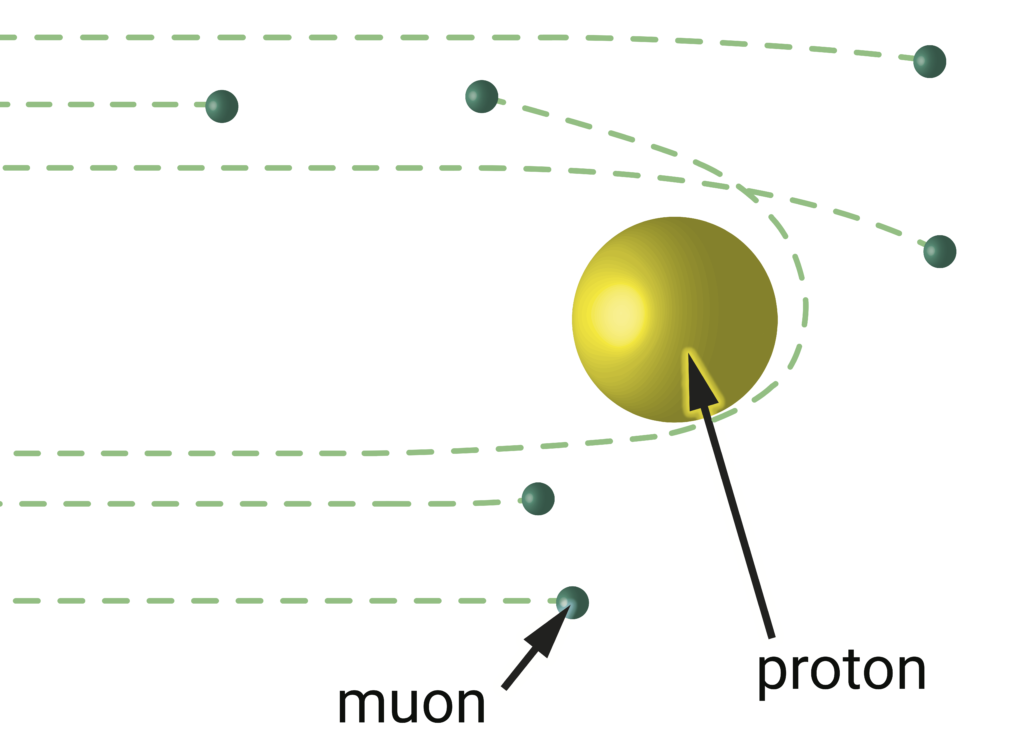 The principle for muon-proton scattering experiments is identical to electron-proton scattering. Differences in the results should only arise if an anomalous force affects the way muons (green) interact with protons (yellow) differently than the way electrons interact with protons. Image credit: Lauren Borja, BSR design team.
The principle for muon-proton scattering experiments is identical to electron-proton scattering. Differences in the results should only arise if an anomalous force affects the way muons (green) interact with protons (yellow) differently than the way electrons interact with protons. Image credit: Lauren Borja, BSR design team.
A new linchpin in this whole muddle is slated to come in 2018. While hydrogen measurements employing electrons have a direct counterpart in the muonic hydrogen measurements, the electron-proton scattering experiments have no muonic counterpart. To the rescue comes the MUon Scattering Experiment (MUSE), currently under construction in Switzerland. MUSE will involve bombarding the proton with muons in much the same way as the electron-proton scattering experiments. Researchers working on the project will also redo the electron-proton scattering measurements in the same apparatus to improve upon previous results and serve as calibration between the electron and muon experiments. Will MUSE also show that the muon delivers smaller radius for the proton than the electron? If so, what hints will it provide for why? The experiment will begin test runs this year and is projected to produce its first data next fall.
As you hold your breath in wait for new results, consider the oxygen molecules you’ve inhaled. At the center of each one of those sextillion oxygen atoms sits a bundle of eight protons. Just exactly how big are they? Answering that question some day might hold the key to resolving some of natural world’s biggest unsolved mysteries. Then again, the puzzle may well be resolved by some yet unimagined systematic effect that ever-so-slightly modifies some of the most precise measurements humans have ever made. The resolution of the proton radius puzzle, whatever it may be, will serve to advance our understanding of this universe and the diverse zoo of effects it sports.
Featured image: The spectroscopy experiments that sit at the heart of the proton radius puzzle rely on strong lasers. By FDominec via Wikimedia Commons.
This article can also be found at Medium.