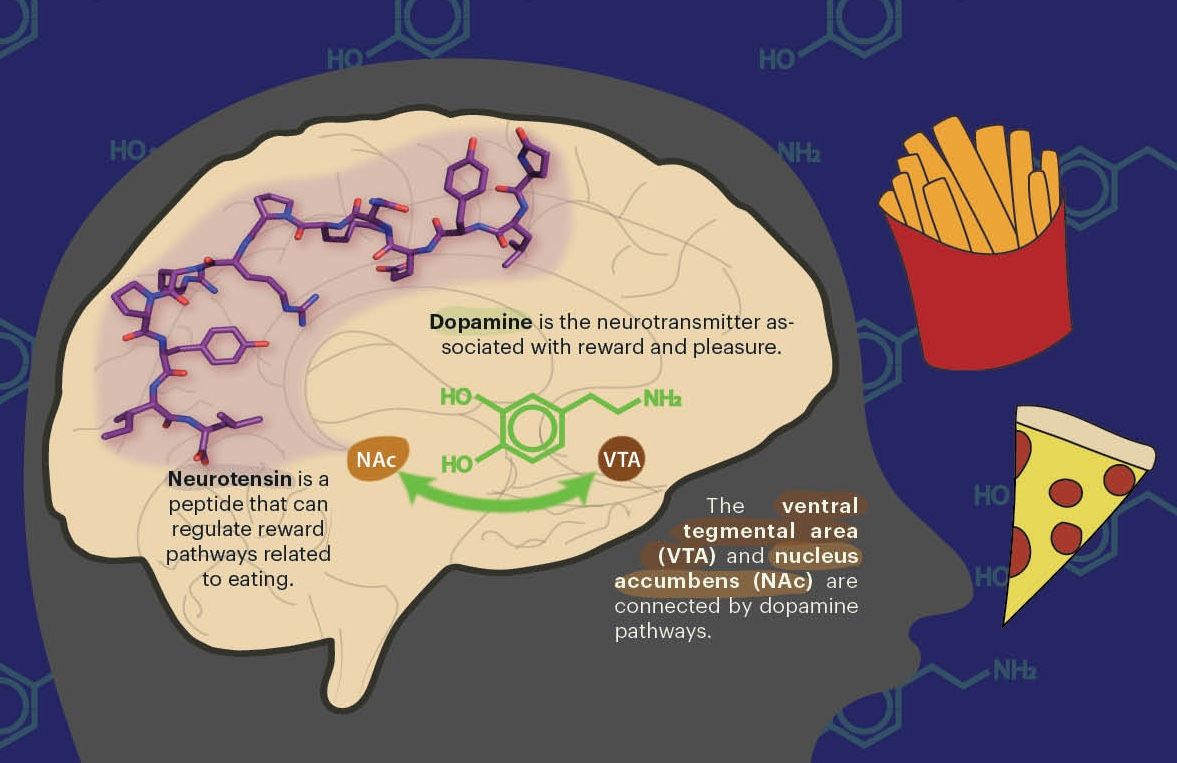
Plastics are everywhere. Yet what they look like at the atomic scale was unclear. Recent work from the lab of Nitash Balsara, professor of chemical and biomolecular engineering at UC Berkeley, has revealed the structure of basic synthetic polymers on the nanometer scale. Polymers are chemicals made of many molecules linked together in chains, and man-made polymers are versatile materials used in a wide range of products, including polypropylene and nylon. However, much about the structure of synthetic polymers is not fully understood.
The individual molecules that make up a polymer do not always come together in the same configuration each time one forms, making it challenging to capture a consistent picture under a microscope. To work around this inconsistency, Balsara’s group imaged a simple man-made polymer with a technique commonly used by biologists called cryo-electron microscopy, where the sample is frozen in place and then imaged thousands of times. The images are then compiled to reveal the three dimensional structure of the polymer.
This is the first time the organization of synthetic polymers was shown at the atomic level, and although they used an uncomplicated polymer that forms in only two dimensions, Balsara’s group still identified 35 different classes of formations.
Going forward, a more complete picture of polymer arrangement will further our understanding of their chemical behavior and improve polymer design for practical applications like lithium batteries. Because, as Balsara remarks, “in the end, all material properties depend on how the atoms are arranged.”
 A simplified version of the polymer structure discovered by Berkeley scientists.
A simplified version of the polymer structure discovered by Berkeley scientists.
Hayley McCausland is a graduate student in molecular & cell biology
Design: Mackenzie Kirchner-Smith
This article is part of the Spring 2019 issue.