Sana, sana, colita de rana
What frogs can teach us about disease resilience
Two decades ago, the hearts of herpetologists worldwide dropped. New reports announced that amphibian populations, across species, were plummeting. Mass extinction events of this scale are extremely rare, and scientists were skeptical at first—could an entire vertebrate class be wiped off the face of the earth, just like that? But amphibian population decline was soon recognized as one of our planet’s greatest threats to biodiversity.
Surprisingly, after decades of decline, some populations started bouncing back. Understanding what makes some amphibians resilient to disease may teach us valuable lessons about how all living systems can fight emerging diseases and other major global stressors. That’s where collaborative science comes in.
The call for answers
Nearly 20 years ago, Dr. Erica Bree Rosenblum, now a professor of Integrative Biology at UC Berkeley, observed these massive amphibian declines in real-time as a graduate student collaborating with Dr. Jim Collins, an evolutionary ecologist at the University of Arizona. “What brought us all together,” she recalls, “was that amphibians were disappearing. We were all trying to solve a shared mystery.” They thought that things were going to keep getting worse, but they were surprised when the amphibians unexpectedly began to recover. Rosenblum explains, “[As a field] we realized that the next big collaborative project we needed to do should be focused on resilience and recovery, rather than declines and extinctions.”
Since amphibian decline first rang the alarm in the 1990s, one major culprit has come into the spotlight: chytridiomycosis. Also called chytrid disease, chytridiomycosis is caused by a fungal pathogen (Batrachochytrium dendrobatidis) wreaking havoc on hundreds of amphibian species worldwide. The Resilience Institute Bridging Biological Training and Research, or RIBBiTR, was founded by Rosenblum and her collaborators to unite amphibian decline researchers across nine institutions. This project, recently awarded a $12.5 million National Science Foundation (NSF) grant, is bringing together multiple generations of scientists to study mechanisms of disease resilience, using chytrid disease as a model. RIBBiTR represents everything from genetics and immunology to mathematical modeling and ecology. The project’s goal is to understand how amphibian species are starting to recover from chytrid disease, and what that might tell us about the ability of other species to recover from their own ailments.
RIBBiTR is not the first institute of its kind—herpetologists have been collaborating with other researchers to study amphibian decline since the problem was first recognized. In the late 1990s, Dr. Collins and a small group of like-minded researchers received NSF funding for an academic workshop on amphibian decline. Together, they summoned herpetologists from across the world to come together and ask: Are amphibians really under threat of extinction? If so, why? And what can we do to stop it?
At first, it was all a mystery. Habitat destruction, one obvious contender, was ruled out as the primary problem early on—population declines were observed worldwide, including on protected reserves. More insidious, complex, and subtle forces had to be at play.
The workshop, alongside the emergence of the internet and modern global communication networks, connected star researchers across the amphibian science universe. The Environmental Protection Agency covered toxic chemicals while virologists and mycologists, who study viruses and fungi, respectively, tackled infectious disease. NASA studied climate change while conservation biologists investigated habitat change. After years of interdisciplinary work, one particularly destructive culprit stepped into the spotlight: the aforementioned fungal pathogen, B. dendrobatidis.
Under an electron microscope, a B. dendrobatidis spore looks like a serene, gray orb—not particularly threatening. But these spores are capable of incredible destruction, swimming through bodies of water until they reach the skin of an unsuspecting amphibian. The resulting infection is chytrid disease, and it is every epidemiologist’s worst nightmare: highly infectious, highly lethal, and tricky to fight. Amphibians exposed to chytrid disease develop excessive thickening and shedding of skin, an especially nightmarish set of symptoms for animals who use their skin to absorb nutrients, release toxins, and breathe.
Unlike viruses and bacteria, fungal pathogens are eukaryotes, with organelles and DNA packaged up in a nucleus like members of the animal kingdom. Antivirals and antibiotics target pathogens that are very different from animal cells, making it easier to attack the virus or bacteria without killing the animal’s cells. But any treatment that might kill a fungus is likely to kill parts of the animal’s cells, too. While there are over 100 types of antibiotics, there are only four main classes of antifungals. Fungal diseases don’t yet occupy much space in our collective consciousness, but climate change and globalization are enabling their spread to new environments and pushing these cool-loving pathogens to adapt to warm body temperatures. A highly transmissible fungal disease could pit us against a pandemic that is highly infectious and painful to treat. Understanding how fungal pathogens interact with hosts, and how host populations develop resilience and recover from these outbreaks, will help us prepare for the worst-case scenario.
100-year-old frogs reveal hidden truths about the present
There are three main players in any disease system: the host, the pathogen, and the environment. If any of those players makes a sudden move, the disease changes. Major global phenomena like climate change play a big role in shaping disease systems, enabling problematic pathogens to spread to new territory, exposing unsuspecting hosts. But disease evolution implies major genetic shifts in the host, the pathogen, or both.
The Rosenblum lab is trying to understand why evolutionary adaptation is leading some amphibian species to recover, while others continue to struggle. While historically the problem of amphibian decline was mainly being tackled by ecologists, Dr. Rosenblum says, “I was a geneticist by training, so there was an open niche: what can we learn about chytrid disease under the hood, if we marry an ecological perspective with a genetics perspective?” Today, her group is leading RIBBiTR’s genetics efforts with a two-pronged approach, studying the history of chytrid disease from the perspective of both the host and the pathogen.
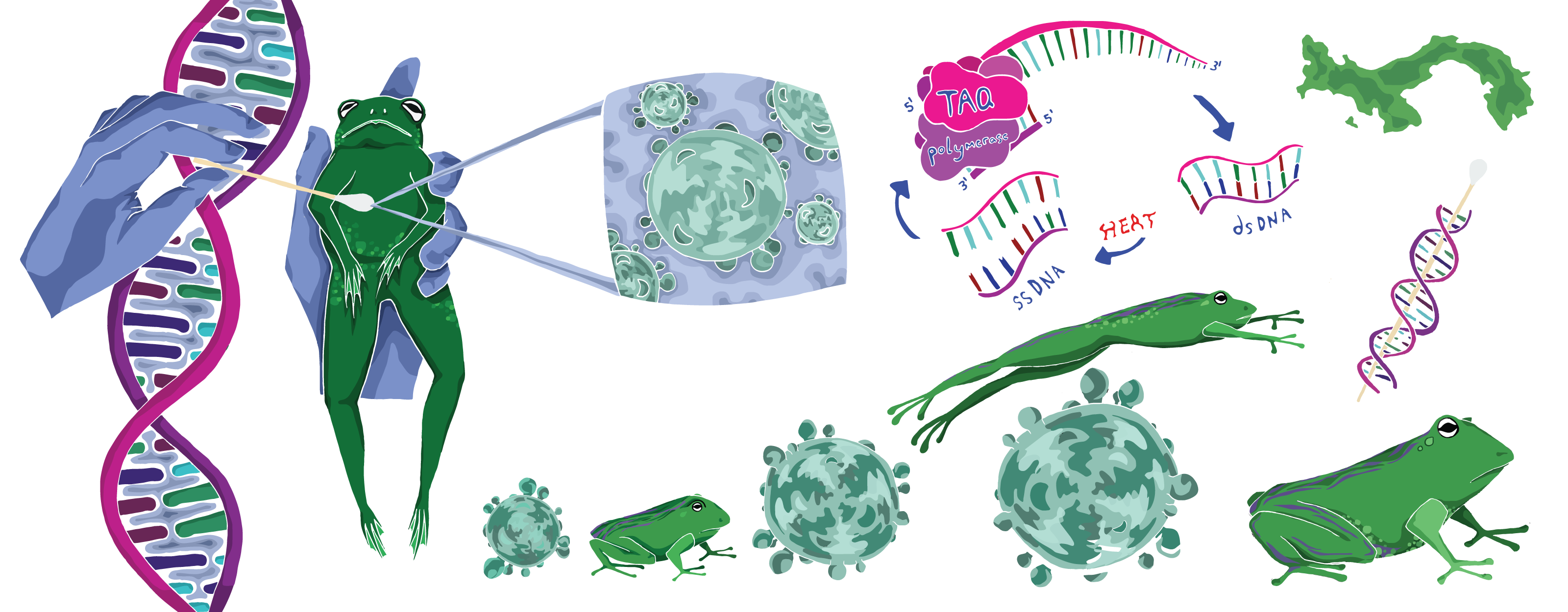
To do this, they sequence host genomes, reading DNA to compare amphibians to one another, and to compare the same host species at different points in time. Scientists trek through the amphibians’ natural habitats and snag genetic samples by swabbing the critters’ belly skin with a Q-tip. This process is easy for the amphibians but presents some challenges for the scientists: samples collected via swabs are often of lower quality than samples collected in more controlled lab environments. To get around this, the Rosenblum lab developed new custom tools to retrieve high-quality genetic data from lower-quality samples, like those collected from decades-old frogs preserved in museums, or from those swabbed at field sites outside a controlled lab environment.
Dr. Allie Byrne, a former Rosenblum lab graduate student, is now a postdoctoral scholar at RIBBiTR who specializes in these techniques. “There are preserved frogs in jars stored in museums all over the world,” she explains, “and we’re swabbing these specimens to see what their genomes were like before chytrid, relative to what they look like now.” To get modern-day samples, she hikes along tropical rainforest streams at a field site in Panama. “It’s day after day of that,” Byrne says. “It’s grueling work, but it’s so fun seeing all the amphibians and really feeling like you’re a part of it.”
As amphibians adapt, so does the disease. The Rosenblum team also runs polymerase chain reaction (PCR) tests on frog belly swabs to find molecules of B. dendrobatidis. To sequence these genomes, Byrne first runs a qPCR (short for quantitative PCR) test, a more informative version of the same PCR tests occupying a lot of space in the public eye over the past two years as one of our best tools for detecting COVID-19. PCR tests amplify DNA, enabling researchers and clinicians to detect even the small quantities of genetic material produced by microscopic pathogens (like B. dendrobatidis and SARS-CoV-2, the virus that causes COVID-19). While a sample from a swab might contain lots of different genetic material from lots of different organisms, scientists use primers—short, single-stranded bits of DNA matching a chunk of the pathogen’s DNA—that selectively target only the genetic material of the pathogen of interest. If a PCR doesn’t amplify any DNA, then the pathogen was probably not in the initial sample. While PCR tests can only show whether a pathogen is present or absent, qPCR also reveals how much pathogen DNA was found.
Byrne uses primers that match B. dendrobatidis. To make sure that they can still spot this genetic material in low-quality samples, like time-worn DNA on the skin of 100-year-old museum frogs, her research group designed special primers that go straight for segments of the B. dendrobatidis genome that are most diagnostic for different lineages of the pathogen. Testing both museum samples and live frogs across the world and comparing genetic data of disease in decades-old and modern-day samples, can reveal the historical emergence and spread of B. dendrobatidis. “We don’t just know whether or not the pathogen was there at a particular place and time,” Rosenblum explains, “but we actually know what strain of the pathogen was there, and how closely it relates to others.” By tracing the waxing and waning of pathogen strains over time, scientists can map out its evolution and better understand how its mutations shaped the history of chytrid disease.
Disease is an ever-moving target
Over two years and multiple variants deep into our own global pandemic, we’ve become intimately familiar with the potential impacts of pathogen evolution. “To me,” Byrne says, “the most interesting parallel with COVID is this genetics question.” When a disease infects a giant population, there’s more opportunity for pathogens to mutate and evolve. Byrne explains, “Just like we’re concerned about whether new variants of COVID are more infectious or more deadly, we’re worried about chytrid evolution.”
To understand emerging infectious diseases, we need to understand the world through which they spread, past and present. “We can’t treat diseases like they just appeared from outer space,” Rosenblum says. “Everybody talks about emerging infectious diseases like they’re one thing, rather than complex organisms with fascinating evolutionary histories. It matters a lot, in terms of how we respond and treat disease outbreaks.”
“Many people would argue that chytrid is the most important infectious disease system that we’ve ever seen, or that’s ever been recorded in the history of understanding diseases,” Dr. Jamie Voyles, associate professor at the University of Nevada, Reno and co-director of RIBBiTR, says. In the past, scientists largely assumed that infectious diseases were not capable of driving a species to extinction. As infectious and deadly as the coronavirus pandemic has been for humans across the world, we’ve never feared our literal extinction. The understanding was that, as potential hosts get sick and die, the pathogen loses opportunities for transmission. Eventually, infection rates wind down, giving the host population a chance to catch its breath. Chytrid, and the sheer scope of its destruction, challenged everything scientists thought they knew about infectious diseases.
Learning from amphibian resilience
The research efforts led by RIBBiTR and its predecessors will not only teach us about amphibian resilience to chytrid disease, but it may give us the key to understanding human infectious disease systems—including the one we’re battling right now. Tackling the chytrid crises required scientists to face just how little they understood about host-pathogen biology and the environment. Using the host-pathogen relationship between amphibians and B. dendrobatidis as a model, scientists began solving basic science questions to chip away at the mystery of amphibian decline and recovery. This story—a specific scientific mission leading to unexpected innovation along the way—is familiar. For example, it took a global coronavirus pandemic to launch mRNA vaccine technology into the public sphere. Likewise, figuring out why amphibians are dying is showing us how to anticipate and react to emerging infectious diseases.
It perhaps isn’t surprising, but the COVID pandemic and the spread of chytrid share an underlying cause: globalization. “There are some foundational similarities in the stressors affecting us,” Rosenblum says. “Fundamentally, it’s about how we handle people and things moving around the planet.” After first being identified in Wuhan, China, the coronavirus hitched a ride with humans traveling worldwide on airplanes and cruise ships, enabling its global spread. Similarly, the global scale of chytrid disease was likely brought on by human-facilitated movement. Dr. Voyles explains, “Amphibians are actually moved around the world, and have been since the 1950s, for all kinds of things, like pet trade, food consumption, and science education.” A 2009 Science article reported nearly 28 million live frogs (62% of which tested positive for B. dendrobatidis) were traded globally between 2000 and 2005. Both COVID and chytrid are emerging infectious diseases that came about because of the human-facilitated movement of pathogens around the world. So, perhaps the same mitigation techniques—frequent testing, quarantines, limiting transmission, travel restrictions—may help both us and amphibians get through our respective crises.
It’s difficult to imagine now, but pandemics end. Even at a much shorter timescale than evolution affords, Voyles points out, “We have great hope, in that we have an immune system with the ability to learn to recognize pathogens.” We don’t necessarily need to wait for evolution to run its generational course—as we’re exposed to variants of a pathogen, like SARS-CoV-2, our bodies learn to adapt and respond.
Pandemics are also unpredictable. Rosenblum takes the unexpected post-chytrid recovery of some amphibian species as a good sign for us, too. She says, “There are some rays of hope, even though we don’t have the benefit of hindsight in the middle of this human pandemic. Things do change. Hosts evolve, pathogens evolve.” Part of what’s been so fascinating about studying chytrid over the past 20 years, she adds, “is how unexpected it’s been. We couldn’t predict the outcome or trajectory of the disease systems we studied from what we knew when the pathogen first arrived.”
Given our current media landscape, parallels between chytrid and COVID are tough to ignore. However, for conservation biologists, the intrinsic value of understanding amphibian population decline can't be overstated. Chytrid is disturbing the entire amphibian vertebrate class, which includes thousands of species worldwide. As important players in both aquatic and terrestrial environments, losing them will disrupt two entirely different ecosystems.
“There’s inherent value in our world’s diversity,” Voyles says. “There’s definitely a benefit to having all organisms and all species within our world.” It may not be immediately obvious why a threat to amphibian biodiversity also threatens human health, but as Rosenblum explains, “Amphibians are part of the middle layer of the food webs of most ecosystems, and there’s a colossal impact when you lose that whole layer.” Other organisms lose their food sources, still others lose their predators.
Big problems need collaborative solutions
If this disease system and its environment sound overwhelmingly complicated, that’s because it is. Without grant funding for large scale interdisciplinary research institutes like RIBBiTR, studying problems of this global scale is nearly impossible. “We’re studying complex, interdependent, irreducible phenomena that involve many different host species and many different pathogen strains in many different environments across the world,” Rosenblum explains. “Honestly, I think anyone not doing interdisciplinary research on complex biological systems is operating from complete hubris, thinking that they’re going to be able to understand anything themselves.”
By bringing together a community of passionate, like-minded researchers with a broad range of expertise, large, elusive problems can be broken down from all sides. However, Rosenblum argues, it isn’t enough to assemble an interdisciplinary team based on academic specialty alone. “Putting together teams that have complementary expertise and a real foundation of trust is really different from putting together teams that just check the right boxes.” RIBBiTR was founded by a team of people who have been working together, off and on, for 20 years. “Some of us are dear, close friends,” Rosenblum says, “and some of us are each other’s advisors. The sense of trust we’ve built is important. We don’t just need public trust in scientists. Scientists should also feel like they have a base of shared understanding, so they can challenge each other.”
With NSF funding secured, the RIBBiTR team is looking towards the future. Between their collaborators, the institute has field research teams in Panama, Brazil, and several sites in the United States. With swabs in hand, they are ready to uncover the amphibian community’s secret to achieving disease resilience. Data scientists at their home institutions are preparing to discover genomic patterns connecting these global communities. University professors and conservation biologists are preparing coursework and outreach programs to educate people on the importance of biodiversity. There are a lot of pieces to coordinate, but Rosenblum points out, “The reason we study complex things in complex ways is because that’s reality.”
------- Celia Ford is a graduate student in neuroscience
Design by Madeleine Snyder
This article is part of the Spring 2022 issue.