An Interview with Dr. Michael Conboy
Aging is a complex biological process that involves the accumulation of damage in our bodies. If we can understand the biological mechanisms of aging, we may be able to slow down or even reverse its effects, opening the door to extending human life. Over the last decade, there has been a surge of interest in the field of longevity, and researchers at UC Berkeley have made significant contributions to the rapid progress in this field. In particular, the Conboy lab has shown that diluting the blood of elderly mice can rejuvenate them. In this piece, graduate student Deniz Tekalp interviews Dr. Michael Conboy, a research scientist in Dr. Irina Conboy’s laboratory, on his views surrounding longevity research and his current research.
Deniz Tekalp: What is your definition of aging?
Dr. Michael Conboy: It's the extended life of the system to the point where it starts to fall apart and not work as well as it did when it was young.
DT: Are there specific quantitative measures of it? Or is it more like, we know when you see it but it's difficult to measure it?
MC: We know when we see it. If you put two people up on stage in front of 100 people, and if there's any reasonable difference in their age, most people will be able to tell who's younger and who's older. So as a gestalt, you can see the effects of aging. Everybody says there is this hallmark of aging or that hallmark of aging, and depending on what you're looking at, in your favorite tissue or organ or part of the body, you'll see signs of aging. That's one of the motivations for the research that professor Irina Conboy and I have been doing for the past 20 years. We ask if there's something common among the aging of all the tissues and organs in the body. That has led us to study blood. Although there are other things that are common to tissue, there's vasculature that carries the blood, and then there's the nervous system too.
DT: What is your theory of aging? More specifically, to what extent is it genetically programmed and to what extent is it entropy? Is there a possibility that there's a single root cause? Or is it many different things?
MC: There are a few things in some high-level theories of aging that I have trouble with. The idea that there is a specific program that makes you age and kills you, I think is unnecessary. Such mechanisms exist in nature. And there are examples in biology, that are the exceptions to prove the rule. So there are bugs like mayflies that can run around for a day, mate, and they die. Or salmon may lay their eggs and then die. There might be other reasons, but there seems to be some sort of an on/off switch, that kind of shuts the system off. But for the rest of us, I don't think you need to invoke that. I think there's enough wear and tear, damage and metabolic dysregulation that I think what is more likely is there are mechanisms that keep us young and healthy, and our tissues working properly. And then over time, those mechanisms fail. So you don't need to invoke genetic programming. In fact, if anything, if you look at us compared to our closest relatives, apes, we live longer than any ape. If there's any program that's causing us to grow [biologically] older, that program isn't working as well for us.
When we get to maturity, we stop adding new freshly-minted tissue. Once that happens, it's all up to maintenance. Given that kind of a system, I don't think you need to invoke a programmed theory of aging to grow old. The dysregulation and the accumulated errors of that imperfect system, and not having signals to put on more freshly minted tissue is sufficient. Imagine as an example some animal that gets 50% more organ tissue this year than it did last year. If the old organs are wearing out, the new tissue can take up the slack. What we find is the signals that are involved in growth and making new tissue tend to be rejuvenative and the signals involved in differentiation, especially when they get accumulated, tend to be pro-aging.
DT: There is clearly a programmed development path as we grow into adulthood. So do you think wear and tear kicks in once it stops and there is a phase shift?
MC: I think there's a growth phase where there are signals in the body, for example human growth hormone and growth factors, that are being produced to get more tissue produced. Those tend to encourage growth of the types of cells that do a lot of DNA repair and protein checks. When you get to adulthood, it’s all sex hormones and really no more growth hormone. We’re not growing in height, bones aren’t getting any longer, you’re not putting on any more muscle etc. As you prepare for fertility, that may have its own maintenance. But after that, there’s very few signals to, for example, tell the stem cells to divide and maintain tissue. So the bulk of the burden for maintaining the tissue falls to the progenitor cells that can proliferate, but they don't seem to have the ability to repair DNA and maintain telomeres [like stem cells do]. And maybe that can contribute to the declining function of tissues.
DT: To go back to a point I first raised about whether there are quantitative measures of aging, I want to ask about epigenetic clocks, which have become somewhat popular. Do you think they are a good measure of aging, and whether a reversal in the epigenetic clock can indicate rejuvenation?
MC: I'm not going to talk too much about that because we have a paper we're working on right now that is going to get at some of those questions. One thing I can point out is that the epigenetic clocks are trained for age. So you have epigenetics on one axis and age on the other axis, and then you do some computations to try to get some sort of correlation between them. It’s not surprising that those can measure age because you have huge amounts of data points there. What we’re struggling with and thinking about are the implications.
DT: I'll move on to asking about your research. And your research, as far as I can tell, has this theme about therapeutic plasma exchange and changing the blood signaling environment, and measuring the effects of this on aging. Do you have a high level overview of your research agenda?
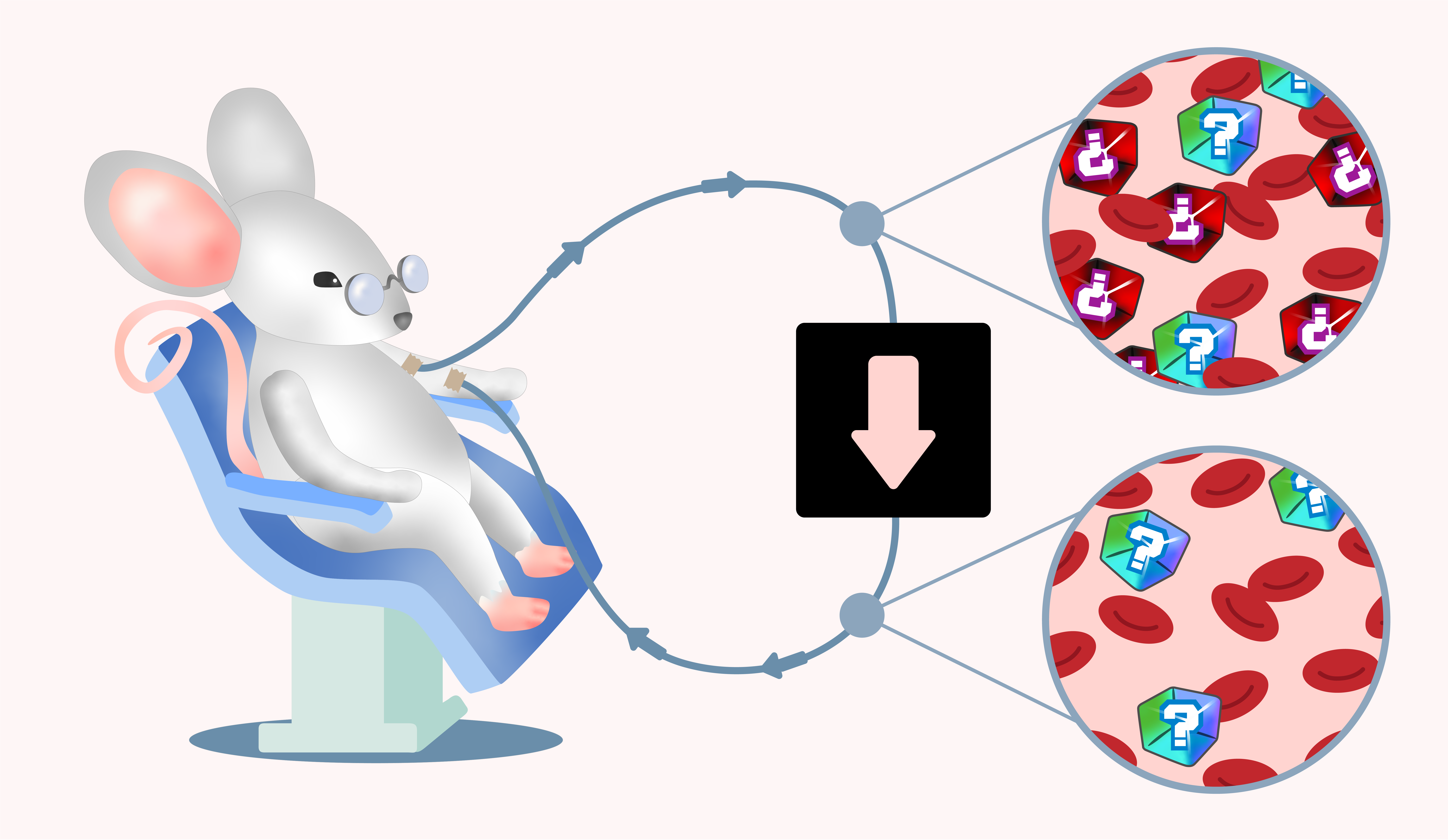
MC: One question is: is there something that circulates in the blood that seems to control or influence aging? To answer this question, we connected mice together through parabiosis.The original idea was to replace the blood of the old mouse with young blood and vice versa. But we couldn't do that, so we did the parabiosis, which showed there's something that's circulating in blood that influences aging. Alternatively, there is the possibility that it was the system of parabiosis that either gave rejuvenation to the old or burden to the young. As a follow up study, we got the expertise and the funding to do blood exchange. For this, it’s just blood that goes back and forth, mice are not being joined, they’re not going to the same food bin, or sleeping in the same nest. The old mouse doesn’t have access to the young kidney and the pancreas and the circulating hormones and vice versa. So it’s just blood, just one time. And that showed that the blood had the bulk of the rejuvenation effect. So is it something in the young blood, or is it something in the old blood?
Next, we came up with the idea of the neutral blood, and that was supposed to be just sort of like a pilot, to rule out the plasma, because most people thought it was white blood cells or platelets, or maybe red blood cells might have oxidative problems. So we threw out the plasma and kept the platelets and red and white blood cells. It turns out this is the influencing factor. So this is the idea of neutral blood exchange by replacing plasma with something that had just albumin and saline [but no signals of any age].
 A group photo of the Conboy Lab in 2018. Dr. Michael Conboy is fourth from left.
A group photo of the Conboy Lab in 2018. Dr. Michael Conboy is fourth from left.
DT: So, the idea is that when you replace the old blood with neutral blood, you get rejuvenating effects. What exactly are the harmful signals in the old blood?
MC: A lot of people have pinpointed those over the past couple of decades. They tend to be things that are associated with inflammation. For example interleukins, tumor necrosis factor, TGF beta BMP group. Others have suggested various smaller peptides and microvesicles. But the big question is, if you have 100 things that change, which of those are more important than others? A lot of these signaling molecules are hierarchical, some are more regulatory than others. And if you can change just a few of those, the thought is then that maybe the rest of the factors will fall in line.
So there's another question, if we start blocking the old signals and augmenting the young signals, can we drop down the dosages but then get combinatorial effects? Does it end up being a more effective, safe, approach? Does it mimic the young state as opposed to throwing a hammer into the old state?
There's a weird observation that we had from the neutral blood exchange that when you got rid of the plasma, you would expect the protein levels would drop in the blood. And immediately they did. But later on, maybe a week later, not all those factors stay low. Some go back up, and some stay low, some are higher than the start. Why is that? There is a rather simplistic model that was in that paper that said, if some proteins repress others and you remove them both, the repressed protein can reset at a higher level. But we are getting into questions that are complicated, and require a lot of thinking and computational assistance to actually work through.
DT: Do these harmful signals have otherwise positive effects? And if I get rid of those positive effects, is there a risk for side-effects?
MC: We are typically looking at signals that were good at their lower levels when we were young that have been important signals since early embryogenesis. But the same signals are now affecting different tissues slightly differently because their genetic and epigenetic state and transcription stages are different. So they perceive the signals a little differently. Also, if you take inflammation and look at those signals, they tell the immune system it's time to go to work. They've been existing in an undifferentiated state, and as a result of some infection or pathogen, it finishes differentiation and goes and attacks that problem. But then that persists. That’s what they call “inflammaging”. But you needed to have had that to fight the infection. The relative levels and the coordination of those signals sometimes get out of whack.
DT: What have you done with human clinical trials to observe these effects?
MC: Well, we haven't done anything in our lab because we're not a medical institution. But collaborators have gotten some blood samples from people that they've done therapeutic plasma exchange with. For this process, you take the patient and you hook them up to a machine that can separate the various components of blood through membranes. You keep the red blood cells, white blood cells, and platelets, but throw the plasma away. Then there’s a big bag of replacement plasma, which is basically just albumin and saline, which comes back in your body. And so you can get to 50, 60, 70, 80 percent replacement of your plasma with purified saline albumin. All the bad stuff that was in your plasma, autoreactive antibodies or complement factors, all get thrown away.
There is a pilot [study] where they're trying this on older people that meet the inclusion criteria. They had to have some age related condition for example, arthritis or bad knees. They look at their blood, and that's where they saw that some factors drop immediately after the exchange, but then a month later, some might be back to where they were, some might still be low, but some might even be higher. There are not many patients they've looked at yet, but they have some tests that they do like urine test and blood test, and they ask questions like how do you feel, has your age related condition improved? The reports I'm getting are encouraging – no one's getting worse and a lot of people seem to be feeling better. I think at this point it could really use a few million dollars of NIH funding to do a proper clinical trial. But it's very encouraging.
DT: What are some of the big open questions that you're thinking about in your research? You've mentioned, trying to understand why some protein levels go up and down. Are there other questions that you're thinking about?
MC: A big question in our lab, we've done these various interventions and we see rejuvenation of old tissue. That sounds encouraging, but does that actually make the animal any healthier in the long run? So the idea of looking at healthspan, how long does the animal stay healthy and does the animal live any longer? There's a big discussion in the field on, are we going to push the maximum lifespan or are we going to compress morbidity so that you have a longer healthspan? So will everyone live to whatever the theoretical maximum is, say 120, yet we're all perky and running around, and maybe you are a little grayer and a little more wrinkly, but still perfectly lucid and functional to 119, and then that last year is just a steep dive off the cliff? Or will it push the maximum age out, say, to 200-250 years? We can answer that in mice. If we're doing things that would translate to people and potentially show improvements of various organs and tissues, that would suggest nice testable hypotheses that we could look at.
DT: As you look at the field and your research, what are some of the key bottlenecks?
MC: I think one bottleneck is coordination of people with different specializations. We are all experts on narrow specializations. Our lab is really good at taking mice and doing neutral blood exchange, catheterization, or other blood manipulations in mice. We're also really good at looking at some of the factors that affect the stem progenitor cells. But we don't have any expertise on doing a clinical trial. One of the things that made bioengineering pop in the last decade is that it brought people with very narrow specializations together to work closely with each other. So I can come up with a question and someone with a very narrow specialization that doesn't even ask that question, but knows how to answer it, may answer it. We start talking and they say, oh, we could answer that question because of this physics or this chemistry. So I think there are solutions to those kinds of bottlenecks, but I think it involves getting people to work and coordinate together.
DT: How can the funding process be improved?
MC: One thing that the NIH could do is try to encourage more collaboration. I think in order to do that, you need to reform the winner-take-all model for funding because it's not the best science that's getting funded, it's the networking, and it doesn't encourage openness and collaboration. It would be better if the prize money was distributed around, which would lead to more labs talking to each other. That's where I think some of the best science comes from, when you have those smart people talking, and not thinking, “I'm not going to say anything until my papers are ready”.
DT: You said your battery's running out and my questions also ran out. Thank you for your time.
------- Deniz Tekalp is a graduate student in industrial engineering and operations research
Design by Meghan Turner