In the fields of observation chance favors only the prepared mind
By Levi Gadye
January 12, 2015
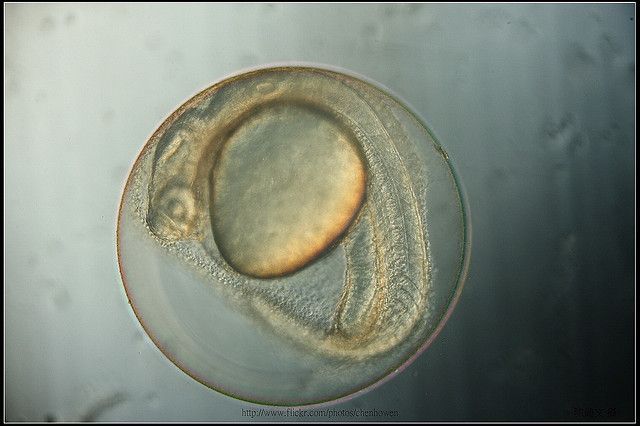
Image: Zebrafish embryo, 27.5 hours post-fertilization. Ho-Wen Chen.
The evolution of vision was one of the most effective advances in the evolution of animal life: the vast majority of animals have eyes, and many animals, humans included, have made vision their go-to sense for perceiving the world. But light perception doesn't always depend on eyes (think of plants growing towards light in dark places). And even neuroscientists can be surprised to find light perception hiding in the most unexpected of places.
Drew Friedmann is a graduate student in Udi Isacoff's lab at UC Berkeley who studies how the spinal cord of zebrafish wires up its neurons during development. One year back, he was hard at work trying to expand on some of the earlier findings of his lab. Using state-of-the-art techniques that allow scientists to observe individual neurons talking to one another, members of the Isacoff lab had identified the window of time in which spinal cord neurons begin to work in synchrony in day-old zebrafish, a first step in the young zebrafish's development of swimming behavior. (Zebrafish are translucent until they are about 5 days old, making observation of their internal neural activity quite easy).
https://www.youtube.com/watch?v=q40dtdkZUN4
Activity of motor neurons in the zebrafish spinal cord, firing in synchrony a mere 18 hours after fertilization. Koichi Kawakami.
The spinal cord, common to all vertebrates, is famous for containing synchronized circuits of neurons known as central pattern generators (CPGs). The spinal CPG coordinates walking in humans, galloping in horses, and swimming in fish – all with merely indirect oversight from the brain.
The Isacoff lab had pinned down the earliest example of a CPG in young zebrafish, by visualizing the emergence of synchronized activity in spinal cord neurons, but as usual, the lab was hungry for more. Friedmann wanted to know how these young neurons pulled off talking to one another at such a young age, when their chemical synapses – junctions between neurons that use neurotransmitters, like dopamine or glutamate, for communication – had not yet formed.
In very young vertebrates, neurons often communicate without neurotransmitters. Instead, the nervous system uses electrical synapses to connect neurons. These simple electrical synapses, also known as gap junctions, are composed of physical tunnels between neurons that allow electric charge to pass directly from one neuron to the next. As a young vertebrate ages, many of its electrical synapses are replaced with more-complicated chemical synapses, which are the primary type of synapse in adults.
To tackle the question of which types of electrical synapses were wiring up the early spinal CPG in zebrafish, Friedmann began to genetically disrupt the components of gap junctions, in the hopes of preventing the CPG from forming. Gap junctions are made of proteins called connexins, but in zebrafish, there are 37 types of connexins. "I was genetically knocking out the 37 connexins, one by one, but I was at a dead end," he says. No matter which connexin was removed, the fish still developed synchronized CPGs. Friedmann needed more information to figure out how these neurons were communicating.
[caption id="attachment\\_12355" align="alignleft" width="300"][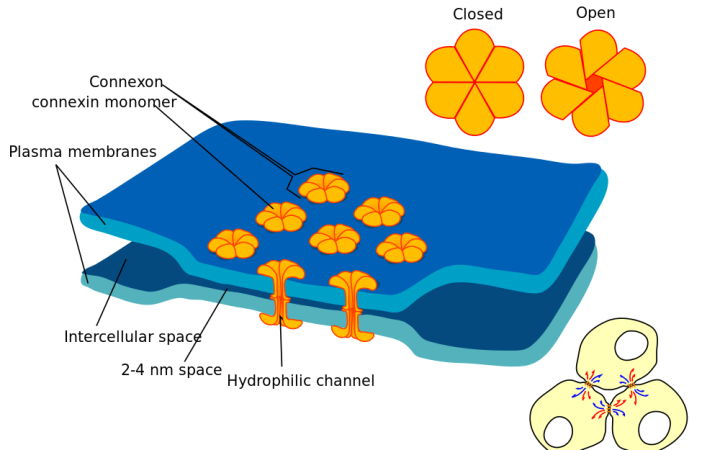](http://en.wikipedia.org/wiki/Gap\_junction) Gap junctions ('connexons') connecting two cells, composed of connexin proteins. Marian Ruiz - Wikimedia Commons[/caption]
He decided to pursue the sometimes-controversial pastime of any biologist who has hit an experimental brick wall: a genetic screen. Instead of starting with 37 connexin genes, he would measure the expression of all the genes that were active in the young zebrafish spinal CPG, and work backwards to figure out which ones were responsible for the CPG's synchronized activity. "We had a genetically-labeled line that included some of the active cell types in the spinal cord at that age," he explains, giving him the ability to physically isolate the CPG neurons from the spinal cord and analyze their gene expression.
Friedmann purified these neurons from the spinal cord and used a technique called RNA sequencing to measure the levels of expression of all of their genes. He then compared the gene expression from these particular CPG neurons to the gene expression of the entire spinal cord, "on a whim" that perhaps the CPG neurons were expressing unique genes (perhaps a particular set of connexins) in order to synchronize their activity at such a young age.
"I was looking exclusively at expression levels of connexins in the active population, looking for ones more highly expressed than in the rest of the neurons," he recounts. "But then I noticed that gene number 16 in the list, really high up, was 'blah blah… opsin.'"
Something clicked. Opsins are the proteins found in the rods and cones of our retinas that give us our sense of sight. (Remember, rods and cones are just specialized, light-sensitive neurons). When light hits an opsin in a neuron, it sets off a chemical reaction inside the neuron, which can either excite the neuron (making it send a signal) or inhibit the neuron (preventing it from sending a signal). One-day old zebrafish don't have functional eyes. So what in the world was an opsin doing in neurons in the developing spinal cord?
That day, Friedmann had already booked some time on Rachel, a popular microscope at the Molecular Imaging Center at UC Berkeley, for "some other experiment for the connexin project that didn't work out." Now determined to figure out why a light sensor was present in the spinal cord CPG of day-old fish, he began flashing light on the young zebrafish while simultaneously videotaping the activity of their spinal CPG neurons.
Based on a single paper he had dug up that morning on this particular opsin, Friedmann guessed that the opsin should excite the CPG neurons that contained it in response to light. The day was drawing to a close, though. "I'm in there for 2 hours and I'm not seeing [a light response]," he recalls, "and I'm ready to give up and go home. Then I opened up all my movies and flipped through them one by one, rapidly. And I noticed that right after the light flash, in every single movie, there was never an event."
He continues. "But there was always a little gap [in activity]. I compressed all the videos on top of each other, and then it was really obvious, that there was a gap after each light flash. And that's how I realized it was inhibitory."
Light somehow paused the synchronized activity of the CPG neurons, via this opsin in this spinal cord. Friedmann's bold genetic screen, searching for connexins, had serendipitously uncovered a light response in the spinal cord of day-old zebrafish. But what did this mean?
[caption id="attachment\\_12351" align="alignright" width="203"][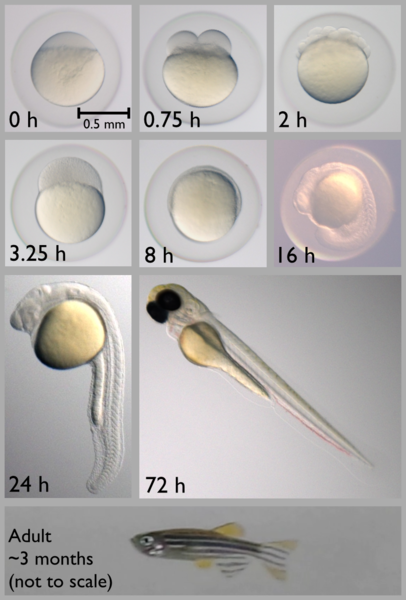](http://berkeleysciencereview.com/wp-content/uploads/2014/12/zf.png) Early zebrafish development, from 0 to 72 hours post-fertilization. Note how young zebrafish remain trapped in their embryonic chorions until about 24 hours. Ed Hendel, Wikimedia Commons.[/caption]
A mere 24 hours after fertilization, young zebrafish spontaneously flick their tails, somewhat akin to the first steps of a young human baby. However, at this age, the fish are often still stuck in a clear sac known as the chorion, so they can't actually swim. These tail flicks inside the chorion cause a behavior known as 'coiling.' Friedmann knew that synchronized activity in the spinal CPG of zebrafish was responsible for this coiling, so the necessary next step, he says, "was a trivial experiment with some day-old fish," which were always available in his lab. Would shining light on young fish - pausing the spinal CPG - stop their coiling behavior?
One last piece of serendipity then fell in place. The Isacoff lab "already had a board of LEDs ready to go" on an array of 48 small wells, each containing a single young zebrafish, for a different experiment. Friedmann's spinal opsin happened to be most-sensitive to light that nearly matched the wavelength of these LEDs (green light of about 504nm). Using this apparatus, he could simultaneously shine the correct wavelength of light to activate the opsin in these spinal cord neurons, and videotape the behavior of all the fish.
"I tried it the next day. That was when I saw it. I'm watching the movie on the screen, I've got 48 fish in the dish, and they're all doing their thing, coiling in the dark," Friedmann says. "Then I turned on the light. Coil coil coi—they stopped. And that's when I realized. I'm going to graduate."
Friedmann's work elegantly showed that light - detected by the spinal cord - could put the brakes on the zebrafish's earliest attempts to swim. Though he had set out to better understand how the spinal CPG becomes synchronized, he had instead stumbled across a non-visual photoresponse in the spinal CPG, a response that directly influenced behavior.
It turns out that non-visual opsins, that is, opsins that do not play a role in vision, do play important roles for other aspects of biology, most famously for setting our circadian rhythms. Beyond that example, though, there are non-visual opsins in the brain, where light is thought to never penetrate, as well as in the heart, lung, and pancreas, amongst other organs. While we know these opsins are present in all these organs, their functions remain elusive. Light perception, it turns out, seems to extend well beyond the eyes.
https://www.youtube.com/watch?v=pblZPn6A4Yk
Spontaneous coiling behavior in a zebrafish embryo, still trapped in its chorion sac. Harold Burgess/Company of Biologists.
In the case of day-old zebrafish, Friedmann can only speculate why the fish stop coiling in response to light. Perhaps this reflex prevents them from revealing themselves to predators in the daytime, at an age when they are still confined to their chorion sacs, unable to escape. Cloaked in the darkness, or in the shadows of a lily pad, the fish are safe to practice their tail flicks as much as they please.
But beyond this discovery itself, Friedmann's journey from connexins to spinal opsins shows that, even in this day and age, it can be tough to predict what a scientist is going to find when digging into some well-defined problem, like synchronized activity in the spinal cord. Scientists are used to experiments turning up empty, but every now and then, they unexpectedly strike gold (and live for those moments). Over a hundred years ago, Louis Pasteur summed up the need for scientists to confront the uncertainty of science head-on with this timeless quote:
"Dans les champs de l'observation le hasard ne favorise que les esprits préparés."
"In the fields of observation, chance favors only the prepared mind."
So if you run into a discouraged scientist as the new year commences, remind her that chance has noted all her hard work and preparation, and that even in the 21st century, it's still possible to accidentally discover as crazy a thing as a light-sensitive spinal cord in a day-old zebrafish.



